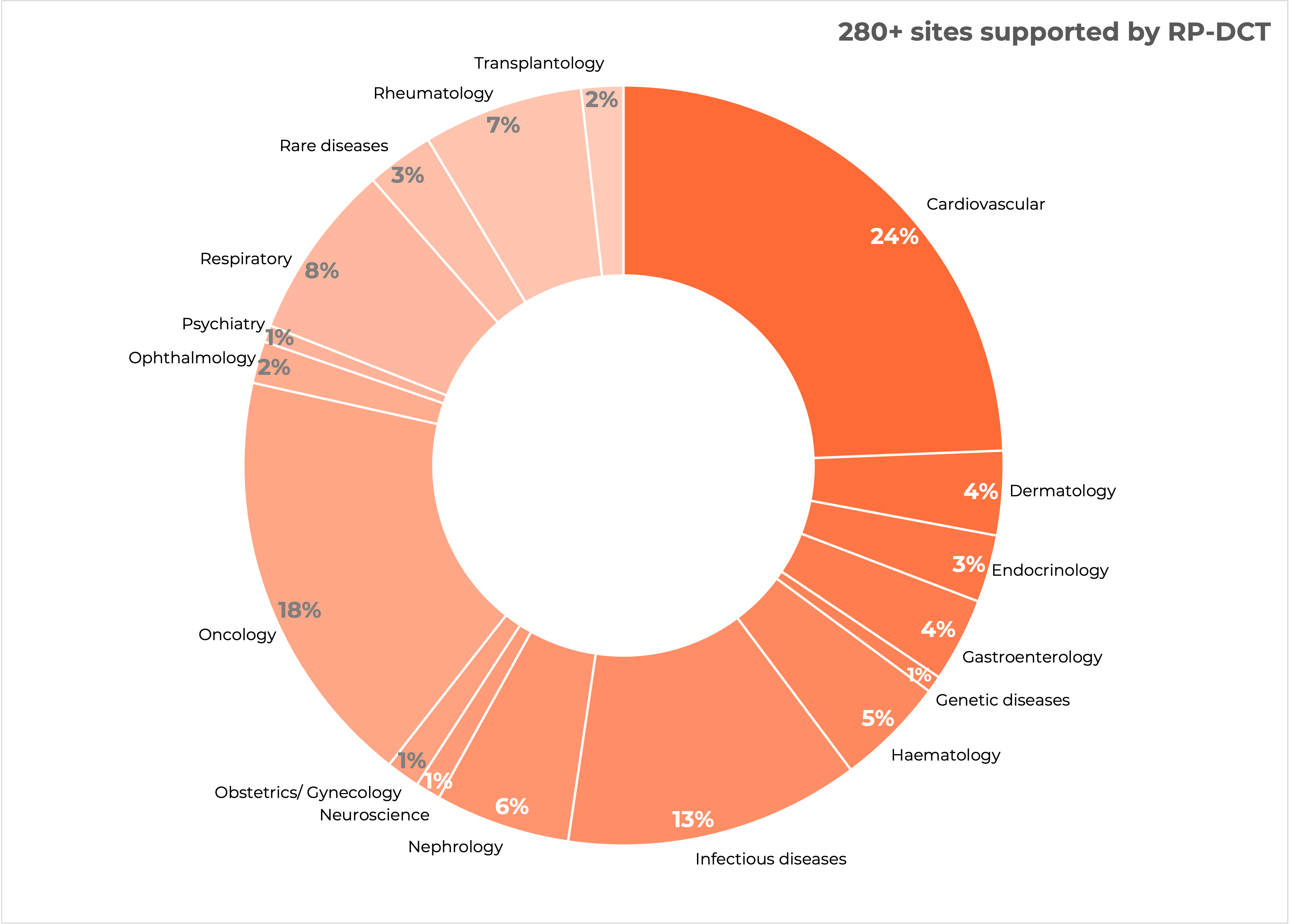
Research Professionals DCT is a leading Decentralized Clinical Trial (DCT) Service Provider in Europe and fully GCP compliant
Research Professionals DCT has expanded its Decentralised Clinical Trial services beyond Europe into the Middle East and North Africa (MENA) region, including KSA, UAE, Egypt, and Morocco.
This milestone expansion supports sponsors and CROs in delivering patient-centric, compliant, and culturally aligned decentralised research across Europe and the MENA region.
Through its integrated digital solutions and experienced research nurse network, RP-DCT ensures seamless coordination of home-based trial activities while maintaining the highest standards of quality, data integrity, and regulatory compliance. RP-DCT’s approach makes clinical research more accessible, inclusive, and efficient across Europe and MENA.
Recruitment is slow. Populations are dispersed. Keeping patients engaged over time is a constant challenge.
At Research Professionals, we manage and deliver rare disease studies using tech-enabled tools that reduce site burden and make participation easier for patients.
As part of our offering, we provide:
- Local-language patient landing pages to support recruitment and retention
- ePRO and wearables for real-time remote data collection
- Medication and visit reminders to encourage protocol adherence
- Integrated visit scheduling and for virtual assessments
- A patient safety alert system for rapid issue escalation
These tools are embedded in our delivery model, enabling efficient, patient-centric studies across geographies. If you're designing a rare disease study and want to make participation simpler without compromising data quality, we’d be happy to show you what’s possible.
If you’re designing a rare disease study and want to make participation simpler without compromising data quality, we’d be happy to show you what’s possible.
Does It Have to Be More Expensive to Bring Clinical Trials to Patients’ Homes?
Decentralized clinical trials (DCTs) often raise concerns about cost. However, with the right approach, bringing trials to patients' homes can be both cost-effective and efficient.
Affordable Solutions Through Strategic Networks
At Research Professionals, we leverage a network of home nurses across Europe, focusing on regions with lower living costs to deliver high-quality care at reduced rates. This geographic strategy minimizes expenses while maintaining excellence in patient care.
Streamlined Trial Design for Savings
By combining affordable home nursing services with fewer clinic visits and advanced technology, we create trial designs that reduce site management costs and patient travel expenses.
Enhancing Recruitment and Retention
DCTs simplify participation by reducing the need for frequent site visits, boosting recruitment and retention rates. Our personalized nurse support ensures patients feel valued, reducing dropout risks and incomplete data.
Inclusive and Diverse Participation
By enabling remote participation, DCTs expand access to diverse populations, enhancing the generalizability of study results.
Cost-Effective Technology Platform
Our digital platform integrates pre-screening, connected devices, role-specific dashboards, offline access, and regulatory compliance — all at a competitive price point.
DCT Medical Research Nursing that brings the study to the subject
Established in 2014, Research Professionals DCT is an industry leader dedicated to facilitating and managing Decentralized Clinical Trials (DCTs). Our extensive network across Europe uses a retained medical research nursing model that can bring the convenience of decentralized clinical trials directly to subjects' homes without compromising the integrity or quality of the clinical research study. Our specially trained medical research nurses follow GCP processes contributing to patient safety, compliance and convenience. Patients and investigators are further enabled with a comprehensive technology suite covering eConsent, ePRO, eCOA, telehealth, vital sign wearables, medication & visit reminders etc.
Putting the D in DCT
Dynamic
We use flexible, subject-friendly, technology-driven approaches, where decentralized clinical trial (DCT) elements can be adjusted in real time to optimize study outcomes and improve patient experiences.
Determined
We use careful planning and decision-making processes that shape every aspect of every decentralized clinical trial, each with a strong emphasis on using remote methods and technologies to conduct the trial in a patient-centric and efficient manner.
Devoted
We are deeply committed to providing exceptional subject care via our team of medical research nursers that prioritizes the well-being of patients, while protecting their privacy, confidentiality and health.
Daring
We deploy the latest technologies and treatments within a framework of evidence-based medicine to provide more efficient and effective decentralized clinical trial (DCT) services. Our innovative approaches are carefully designed to always maintain quality standards while driving improved performance for our sponsors and patients alike.
Diligent
We strive to deliver reliability, integrity, and ethical conduct in every decentralized clinical study we manage, while also embracing the advantages of remote and patientcentric clinical study trial methodologies.
Facts & Figures