How to Reduce your Clinical Study Budget with Central and Eastern Europe
Clinical research studies for pharmaceuticals and medical devices are complex and expensive programs. Every study can be so critical that running a good one can make the difference between your product failing or making it to market. Working with large high-quality clinical research service providers in established western markets can be very expensive to achieve high-quality study programs.
Some clinical research service providers located in Central and Eastern European (CEE) markets can offer all the same high-quality services, access to strong Principal Investigators (PIs), large subject pools, and globally recognized regulatory oversight but can deliver notably lower program pricing without sacrificing any of the study quality. Research Professionals is a leading clinical research service provider based in Hungary with operations across five CEE nations.
Clinical research conducted in CEE countries have earned one of the strongest records with U.S. FDA audits. U.S. FDA audits of clinical research studies conducted in CEE countries have resulted in No Action Indicated (NAI) status 48% of the time between 1994 and 2004. By contrast, only 38% of these studies audited by the U.S. FDA achieved NAI status when conducted in the United States and only 32% when conducted in western Europe.i This data demonstrates that even though the clinical research industry was only emerging in CEE countries that they had very favorable quality indicators even when compared against larger, more established clinical research markets.
As a Central and Eastern European clinical research service provider, Research Professionals recently performed a comparative analysis of the benefits of working with clinical research service providers in the region, with a focus on potential cost savings. Their findings were that a sponsor can realize savings of as much as 45-55% by using a CEE based clinical research service provider over those based in Western Europe or the United States without compromising on the quality of the study. This paper will walk through some of the main clinical study budget line items and identify key areas where sponsor organizations can maximize their cost savings when working with clinical research service providers based in Central and Eastern Europe, such as Research Professionals.
About Research Professionals
Research Professionals (RP) is a leading GCP compliant clinical research service provider based in EU member state Hungary with operations in Poland, Czechia, Romania and Bulgaria, serving customers from across Europe and the globe. Research Professionals has built modern clinical infrastructure, developed leading expertise, draws from a base of highly qualified Principal Investigators (PIs), and large available subject pools to accelerate subject enrolment. With more than 150 experienced and dedicated staff, Research Professionals brings proven experience from managing over 50 major studies in more than 15 therapeutic areas. As Research Professional's operations are based in EU countries, they are subject to EMA regulations and quality standards, its services meet the quality standards of the world's leading clinical research markets.
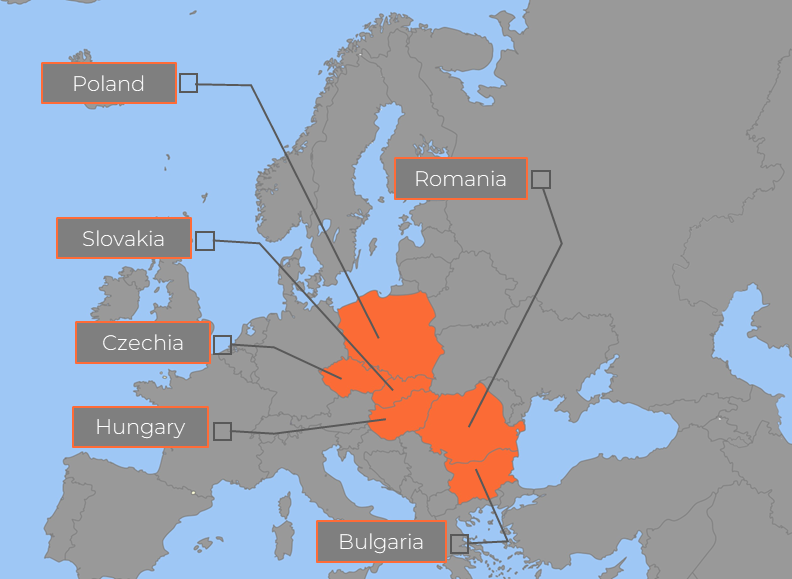
Figure 1 – Research Professionals Countries of Operations Map
Central and Eastern Europe (CEE) clinical research service provider Background
The CEE market for clinical research has grown substantially in the last 30 years. More than 1,500 pharmaceutical studies are being conducted in CEE countries annually, with the largest share, representing over 1,100 studies, registered in Hungary and Czechia.
Clinical Research Study Background
Clinical studies are a large and increasingly complex part of the drug and device development processes. The number of procedures and the parts of trial protocols have increased substantially over the past decade. The number of total endpoints in typical Phase I and Phase II protocols has increased by 27% over this period and the total number of procedures (including routine exams, blood work and x-rays) has increased by 44%. Phase III studies now collect an average of 3.6 million data points per protocol, which is triple the number compared to studies ten years ago.ii Highly complex and data driven studies require costly but robust study management services to achieve their goals than ever before.
As of January 2021, it was estimated that there are approximately 7,800 products in clinical development globallyiii. This translates to thousands of clinical studies being conducted around the world at any time, many of which include the participation of clinical research service providers. These products in development are, however, subject to a very high failure rate. In the United States, it is estimated that only 12 percent of the investigational compounds that reach clinical trials are ever approved for market by the U.S. FDA.iv This high failure rate contributes to the incredibly high average cost to bring a new drug to patients. This is estimated to average as high as $2.6 billion per product over the last decade.v
For those that do successfully navigate through the process, it typically takes 10-15 years to bring a molecule from lab to market. A big portion of the budget for every drug is spent, over the lengthy development timeframe, on clinical research activities. Clinical research studies are a focal point for the drug development process and present multi-variant challenges that can be overcome by the experience and skills of the right clinical research service provider. Working with a clinical research service provider that is able to develop a well devised study plan with a realistic budget, can help advance your molecule to the next stage of development or commercialization. Having a solid budget in place doesn't mean you won't have any unanticipated costs. These can include costs associated with protocol amendments, slower than expected enrollment, or higher levels of subject screening failures than anticipated. You need to build a practical budget that includes funds to address some of the unexpected. Working with a CEE based clinical research service provider that can reliably reduce your costs, while maintaining timelines and study quality can prove a game changer for sponsor companies.
Overall Clinical Study Costs by Phase
As one moves through the various clinical phases, clinical studies become larger and so do their costs. The following data represents U.S. study average costsvi from 2014, which can be considered a conservative estimate for the current costs of U.S. market clinical research studies. Small Phase I studies can range from $4.2 to $5.2 million dollars (depending on the molecule being studied) and as much as $11.5 to $52.9 million for large Phase III studies. NDA reviews can add an additional $2 million per review. Phase IV studies conducted post-approval can cost as much as $72.9 million, eclipsing even the costs of large Phase III studies.
Overall Clinical Study Costs by Therapeutic Category
The therapeutic indication being studied can also have a huge impact on the cost of the study. A review of average study costs by therapeutic indication across all clinical phases have revealed that respiratory products have the highest average study costs, closely followed by pain/anesthesia and oncology. The therapeutic categories with the lowest average study costs across all clinical phases include genitourinary system, dermatology and central nervous system (CNS).
| Rank | Therapeutic Category | Phase I | Phase II | Phase III | Subtotal | FDA NDA/BLA review |
Phase IV | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | Respiratory System | $5.2 | $12.2 | $23.1 | $40.5 | $2.0 | $72.9 | $115.4 |
| 2 | Pain & Anesthesia | $1.4 | $17.0 | $52.9 | $71.3 | $2.0 | $32.1 | $105.4 |
| 3 | Oncology | $4.5 | $11.2 | $22.1 | $37.8 | $2.0 | $38.9 | $78.7 |
| 4 | Ophthalmology | $5.3 | $13.8 | $30.7 | $49.8 | $2.0 | $17.6 | $69.4 |
| 5 | Hematology | $1.7 | $19.6 | $15.0 | $36.3 | $2.0 | $27.0 | $65.3 |
| 6 | Cardiovascular | $2.2 | $7.0 | $25.2 | $34.4 | $2.0 | $27.8 | $64.2 |
| 7 | Endocrine | $1.4 | $12.1 | $17.0 | $30.5 | $2.0 | $26.7 | $59.2 |
| 8 | Gastrointestinal | $2.4 | $15.8 | $14.5 | $32.7 | $2.0 | $21.8 | $56.5 |
| 9 | Immunomodulation | $6.6 | $16.0 | $11.9 | $34.5 | $2.0 | $19.9 | $56.4 |
| 10 | Anti-Infective | $4.2 | $14.2 | $22.8 | $41.2 | $2.0 | $11.0 | $54.2 |
| 11 | CNS | $3.9 | $13.9 | $19.2 | $37.0 | $2.0 | $14.1 | $53.1 |
| 12 | Dermatology | $1.8 | $8.9 | $11.5 | $22.2 | $2.0 | $25.2 | $49.4 |
| 13 | Gerintourinary System | $3.1 | $14.6 | $17.5 | $35.2 | $2.0 | $6.8 | $44.0 |
Cost Impacts by Budget Line Items
The two main categories of any clinical research budget are pass-through costs (PTC) and clinical research service provider service costs. PTCs consist of things like lab vendors, study supplies and travel. Services consist primarily of services that the clinical research service provider directly performs including site management and monitoring, data analysis, project management and submission support. Let's review how working with a clinical research service provider based in Central and Eastern Europe can help to reduce your costs for these critical budget elements. We'll detail some of the key line items in each category and overall cost saving strategies when partnering with clinical research service providers based in CEE countries.
Pass Through Costs (PTCs)
- Subcontracted Vendor costs - These are costs from companies involved in the provision of services such as central testing labs, clinical material depot networks, Electronic Patient Recorded Outcomes software (ePRO). Using local central laboratories in Central and Eastern European countries can represent significantly lower costs for the testing of subject samples with very high levels of service, quality, and compliance with international regulations. These services can result in cost savings in the range of 40% to 60% as compared to comparable services in established western markets. These savings are primarily driven by lower overhead and labor costs in these markets. Additional savings can arise from other outsourced services such as the cost of clinical material depots when sourced locally. Overall, these contributions can mean realizable cost savings no matter the size of the study.
- Travel Costs - These costs can become significant, depending on things like the frequency of on-site monitoring visits and proximity to the research sites. In CEE countries of operation for Research Professionals, the sites are typically concentrated in large population centers resulting in minimal travel requirements. For Research Professionals, the proximity of research sites in CEE countries can often be traveled to by car. In some larger countries with sites further away, travel costs may have to include expensive flights and hotel fees (plus lost time). In general CEE travel costs can be much lower than in larger western markets like the United States. For example, average transportation costs in Hungary can be approximately 30% less than in the United States.viii
- Logistics and courier costs, depot services - This includes the cost of supplying clinical trial materials to the study sites as well as the cost of sending samples to central testing laboratories for evaluation. As Central and Eastern Europe is located within the EU, there are no import or customs fees when moving materials and samples between member states. In addition, as CEE countries are in close proximity to one another, the research sites are generally not very far apart, reducing logistics costs overall. The cost of shipping samples and materials in CEE countries can be considerably less than in large markets such as the United States or Germany.
- Regulatory Authorities (RA) and CEC fees - Historically, European regulatory fees were somewhat lower in Central and Eastern European countries than large Western European nations. However, beginning in 2022 regulatory fees have approached harmonization so there is no longer a price advantage in this regard. As all EU member states have all been held to the same regulatory standards by the same agencies, this demonstrates that Central and Eastern European clinical research service providers have an ability to meet the same high standards demanded in every European nation regarding clinical research. Clinical Endpoint Committees (CECs) are centralized decision-making bodies that help to define efficacy and safety endpoints that are scientifically measurable, objective, and valid for a clinical study. A CEC is needed as clinical endpoints are sometimes subjective and the committee helps to independently assess endpoints across study sites based on a data driven analysis to achieve a more consistent endpoint determination for the study. CEE countries have experienced CEE teams available to support these reviews, however there is limited cost savings here.
- Ethics Committee (EC) - An ethics review is a critical part of every clinical research study. An Ethics Committee, sometimes called an Institutional Review Board (IRB) or Ethics Review Board (ERB), reviews study protocols, and subject consenting information to ensure that the study will be conducted ethically, safely and that subjects are provided accurate and clear information so that they can provide informed consent. If the EC review is not conducted in a timely manner, it can significantly delay the start of a study, potentially slowing the progress of the product toward market approval. Having access to knowledgeable local ECs that can review a study quickly can get the study started on time. Because of the long-term growth of clinical research in Central and Eastern European countries, there are established ethics boards possessing knowledge of the local markets as well as international regulations, while having the capacity to review studies in a timely manner. A typical EC review in Central and Eastern Europe can cost as little as US$1,000. In western markets some ECs associated with institutions can have slow turnaround times for research reviews, in some cases taking months to schedule and complete. There are independent ECs that can perform a review in as little as a few days, but their costs can run into many thousands of dollars depending on the study size by comparison. Having reliable ERBs located in Central and Eastern Europe can result in significant cost savings over independent review boards in western markets.
Services
There are the many and varied services provided directly by the clinical research service provider to the sponsor company. This work is conducted on a day-to-day basis and includes activities such as study management services, data collection, and preparing the results for final reports and regulatory filings. These critical activities are at the heart of the success of your studies and can represent some of the largest costs in your study budget. Have a highly efficient clinical research service provider manage your study can help generate measurable cost reductions, resulting in more budget flexibility for your overall drug development program.
- Site management and monitoring - Site management and monitoring represent a large budget line item in the clinical research service provider Services category. This includes clinical research service provider activities such as the cost to manage each clinical research site as well as the monitoring activities. Monitoring costs are impacted by the subject sample size as well as things like the sponsor's requirements for Source Data Verification (SDV) percentage analysis. Working in Central and Eastern Europe can deliver cost savings of as much as 50% through the efficient use of clinical research service provider labor, lower average salaries and reduced overheads at the sites. Having largely centralized subject pools allows for a potential reduction in the total number of sites resulting in efficient monitoring and site management.
- Subject fees - Subject fees are a critical element of any study's budget. Subject fees relate to the amounts you are required to pay the PI for each subject that participates in the study at their study site. The subject fees must be appropriate for the specific market to avoid any perception of influencing study outcomes. Figure 3 shows the average subject fees from several CEE countries as compared to those typical in the U.S. The sample set clearly indicates that subject fees can be significantly lower in Central and Eastern European countries, as compared to those in the U.S. It also shows that Central and Eastern European subject fees are competitive with Russia, which does not enjoy the same benefits or global credibility of being regulated by the EMA. Subject fees in Central and Eastern European countries can be as little as 36.8% of average U.S. subject fees but these savings average about 50%-60% of the U.S. subject fees overall. For studies with a significant number of subjects the overall cost savings can be significant.
Figure 3 – Comparison of Subject Fee by Country as Compared to U.S.
Country Per Subject Fee (USD) % of US Hungary (CEE) $3,900 36.8% Russia $4,300 40.6% Poland (CEE) $5,000 47,2% Czechia (CEE) $5,100 48.1% USA $10,600 100.0% - Electronic Data Capture (EDC), data management and biostatistics - Data management and related activities are of critical importance to data reporting quality for clinical research studies. Clinical research service providers focus resources on capturing, managing, analyzing, and reporting clinical study data in forms that are suitable for use in the sponsor's regulatory submissions. Using current digital and cloud-based tools allows clinical research service providers to capture and process larger quantities of clinical data more efficiently and with less risk of error. Central and Eastern European clinical research service providers typically employ staff with high levels of education that are capable of effectively managing and interpreting the large quantities of complex study data. In addition, most are very comfortable using the latest digital technologies which also contribute to providing greater value for the sponsor. Lower local overhead and pay rate differences, allow Central and Eastern European based clinical research service providers to complete critical data analysis that yield potential cost savings of as much as 40% as compared to western market clinical research service providers.
- Project Management (PM) - These important administrative activities help to drive studies forward, while maintaining study timelines and budgets. PMs working for clinical research service providers in Central and Eastern Europe bring both education and experience to clinical studies. Clinical research service provider Project Managers located in Central and Eastern Europe tend to hold advanced scientific or medical degrees more often than their counterparts in the U.S. or Western Europe. With low turnover rates, the resulting experience and consistency can result in PMs that avoid the pitfalls less experienced PMs may miss in their duties. In addition, their salaries can be substantially lower than their western market equivalents. Hourly wages for Project Managers in Central and Eastern Europe can be as much as 55% less than in the U.S. Please see Figure 5 for a comparison of clinical research service provider Average Salaries by role.
General Contributions to Cost Savings for Clinical Studies Conducted in Central and Eastern Europe
Lower Cost of Living in Central and Eastern Europe
All the above budget line items are impacted by regional differences in employee salaries and cost of living in the countries the clinical research service provider operates. Central and Eastern European countries tend to have a significantly lower cost of living as compared to the United States. Factors such as housing, transportation and food can have a significant impact on cost of living, allowing for lower wages in the region. See Figure 4, which lists the percentage average difference in cost of living in the five CEE states that Research Professionals operate as compared to the U.S. market.
| Country | Cost of living |
|---|---|
| Hungary | 58% |
| Bulgaria | 66% |
| Czechia | 48% |
| Poland | 55% |
| Romania | 67% |
Average Lower Salaries
This lower cost of living in CEE countries generally correlates to lower salaries for clinical research service provider staff, compared to equivalent roles in the United States. Clinical research professionals living in the CEE tend to be highly educated, technically proficient and bring more long-term company experience because of lower levels of employee turnover. This results in having highly experienced and well-trained staff available for lower labor costs. These lower average salaries are in many cases reflected in the lower cost of clinical research service provider Services and pass-through costs offered by CEE based clinical research service providers. The following table shows approximate average hourly pay rates for U.S., Western European and CEE country salaries for some specific research professional roles in clinical research service providers.
| Role | EU | US | % of US | CEE |
|---|---|---|---|---|
| Clinical Trial Assistant | €60.00 | €87.28 | 52% | €45.00 |
| Clinical Research Associate (CRA) | €100.00 | €157.11 | 45% | €70.00 |
| Project Manager | €180.00 | €187.66 | 51% | €95.00 |
Conclusions
In summary, conducting clinical research with clinical research service providers in Central and Eastern Europe can produce a wide range of potential cost savings across many aspects of your study budget. Although some of the contributions to overall cost savings include comparative differences in average professional wages and cost of living in different countries, it cannot be overlooked that clinical research service providers in Central and Eastern Europe provide highly experienced professional staff, global caliber regulatory knowledge and support, and expert PIs with large subject pools that contribute to study efficiencies and time savings.
Although every study is different with different requirements, a rule of thumb to measure potential cost savings by working with a Central and Eastern European clinical research service provider can be as much as 45-55%. All the contributing budget lines discussed in this paper can add up to real cost savings for sponsor companies choosing to work with established clinical research service providers in Central and Eastern Europe such as Research Professionals. For a growing CRO like Research Professionals, it found that operating in Central and Eastern Europe provides its customers with substantial operational benefits, in addition to critical cost savings. Strict adherence to quality standards, local market knowledge and understanding its customer's needs have been essential to their success as a clinical research service provider. Research Professionals is an example of a clinical research service provider positioned to leverage the inherent advantages of the Central and Eastern European market, providing flexible and responsive services ideally scaled to their customer's demands. Quality studies at reduced costs can be a reality in Central and Eastern Europe with the right clinical research service provider partner.
References:
__________________________________
i “FDA Inspections Outside the USA: An Eastern European Perspective”, Applied Clinical Trials, September 1, 2004 https://www.appliedclinicaltrialsonline.com/view/fda-inspections-outside-usa-eastern-european-perspective
ii DiMasi JA, Grabowski HG, Hansen RA. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of Health Economics 2016;47:20-33.
iii PhRMA Report, Innovation in the Biopharmaceutical Pipeline, December 2021, https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/G-I/Innovation_in_Biopharmaceuticals.pdf
iv DiMasi JA, Grabowski HG, Hansen RA. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of Health Economics 2016;47:20-33
v DiMasi JA, Grabowski HG, Hansen RA. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of Health Economics 2016;47:20-33
vi EXAMINATION OF CLINICAL TRIAL COSTS AND BARRIERS FOR DRUG DEVELOPMENT, U.S. Department of Health and Human Services Assistant Secretary of Planning and Evaluation (ASPE), July 25, 2014
vii EXAMINATION OF CLINICAL TRIAL COSTS AND BARRIERS FOR DRUG DEVELOPMENT, U.S. Department of Health and Human Services Assistant Secretary of Planning and Evaluation (ASPE), July 25, 2014
viii https://livingcost.org/cost/hungary/united-states